FOOT AND ANKLE
At TS&B, we deliver specialized procurement solutions focused on foot and ankle. By aligning clinical priorities with strategic sourcing, we help hospitals and health systems access high-quality implants and instruments that drive better outcomes both clinically and financially.
Our evidence based approach streamlines contracting and vendor management, reduces total cost of care, and ensures consistent access to trusted, innovative solutions. We collaborate closely with surgeons, administrators, and supply chain leaders to optimize product selection and support long-term success.
Our mission is simple: empower providers, improve efficiency, and elevate patient care through procurement that’s focused, strategic, and clinically aligned.
Medical Device
TS&B delivers a focused portfolio of foot and ankle medical devices designed to support clinical precision, streamline procurement, and improve patient outcomes. Our product offerings reflect our mission: empower providers, improve efficiency, and elevate patient care through procurement that’s focused, strategic, and clinically aligned.
Our Product Specialties Include:
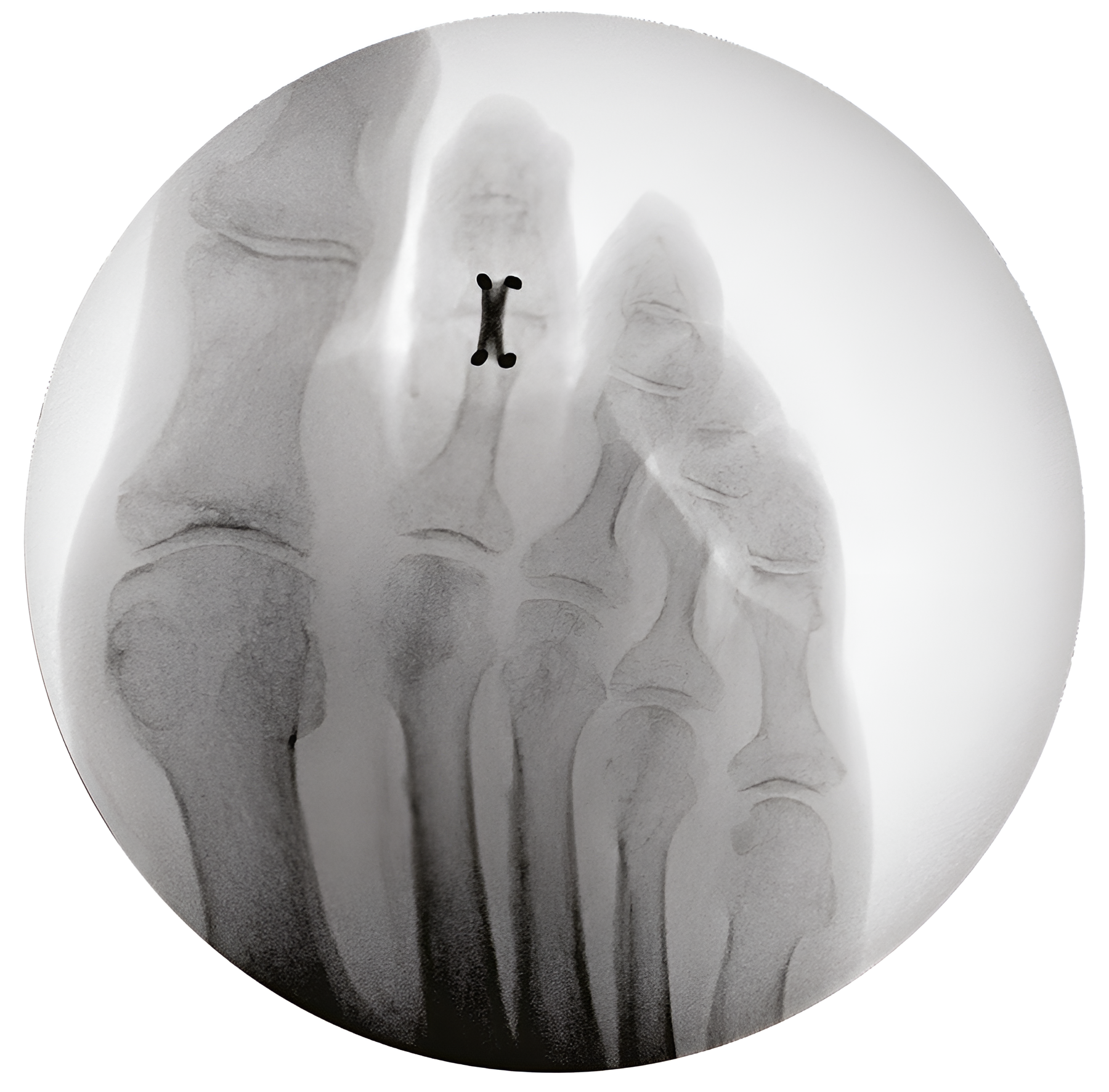
Hammertoe Implants
Designed for flexible and rigid deformities, our intramedullary and PIP joint implants support durable toe alignment and rapid recovery.
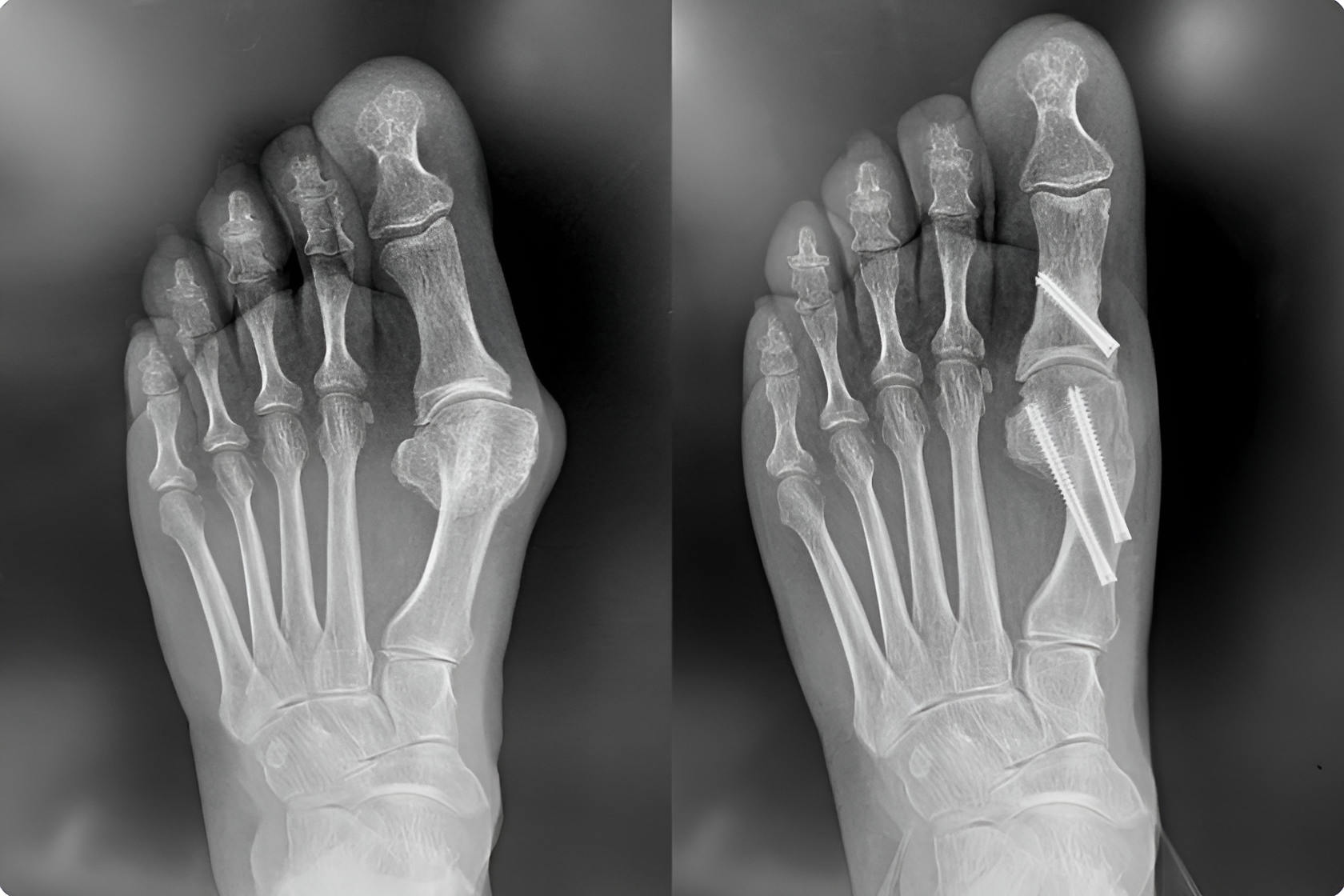
Bunion Correction Plates & Screws
Engineered for hallux valgus correction, our plating systems ensure stable fixation and anatomical realignment for a range of bunion procedures.Arthrodesis Implants (Screws, Staples, Plates)
Our fusion hardware solutions provide structural integrity and compression for midfoot, hindfoot, and ankle joint stabilization—supporting long-term success in complex cases.Cannulated Screws & Headless Compression Screws
Precision-designed for small bone fixation, these implants offer strong, minimally invasive fixation for fractures and osteotomies in high-demand anatomical areas.Biologics
From bone graft substitutes to acellular dermal matrix, our biologic options enhance fusion rates, support tissue regeneration, and complement hardware-based repair.
Regenerative Solutions
-
Our regenerative solutions are exclusively sourced from AATB-accredited providers and derived from donated human tissue that meets the highest safety and quality standards. Prior to processing, all donor tissue must pass rigorous FDA screening and comply with the stringent protocols established by the American Association of Tissue Banks (AATB).
-
Our regenerative solutions are manufactured using tissue sourced exclusively from U.S.-based donors.
-
All donor tissue used in our regenerative solutions is ethically sourced through Tier 1 Organ Procurement Organizations (OPOs), recognized for their superior recovery practices, regulatory compliance, and consistent performance in meeting the highest standards of donor care and tissue quality.